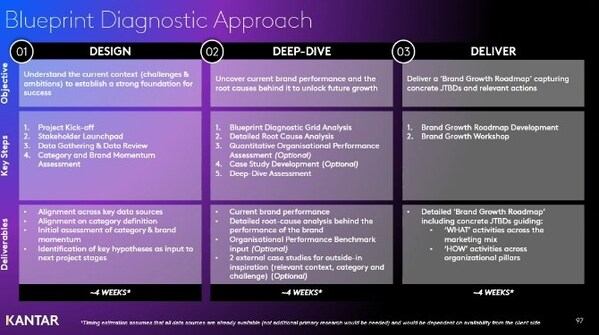
|
- Data spans eleven presentations across both solid tumors and hematologic malignancies, providing added understanding of the potential benefit these therapies bring to patients
- ORSERDU data reinforce its key role in the treatment of ER+, HER2- advanced or metastatic breast cancer (mBC) tumors that harbor ESR1 mutations, while showing initial data from combination trials
- ELZONRIS presentation includes updated real-world data from patients with Blastic Plasmacytoid Dendritic Cell Neoplasm
FLORENCE, Italy and NEW YORK, Nov. 21, 2023 /PRNewswire/ -- The Menarini Group ("Menarini"), a leading Italian pharmaceutical and diagnostics company, and Stemline Therapeutics, Inc. ("Stemline"), a wholly-owned subsidiary of the Menarini Group focused on bringing transformational oncology treatments to cancer patients, announced today that they will present new data related to ORSERDU® (elacestrant) and ELZONRIS® (tagraxofusp-erzs) in two upcoming congresses.
ORSERDU, a once-daily oral endocrine monotherapy, for the treatment of postmenopausal women or adult men, with ER+, HER2-, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy, was approved by the U.S. Food and Drug Administration in January 2023 under its priority review and fast track designation. In September 2023, ORSERDU was approved by the European Commission.
Updated data, including a spotlight discussion on additional biomarker and clinical subgroup analyses from the Phase 3 EMERALD trial, along with new safety updates evaluating elacestrant in multiple combination settings, will be presented at the San Antonio Breast Cancer Symposium (SABCS), December 5-9, 2023.
ELZONRIS was approved by the FDA in December 2018 for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adult and pediatric patients two years and older, in both treatment-naïve and previously-treated populations. In January 2021, ELZONRIS was approved by the European Commission. Updated data, including an oral presentation of updated real-world results in treatment-naïve patients with BPDCN demonstrating durable outcomes and a manageable safety profile leading to prolonged survival, will be presented at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition in San Diego, December 9-12, 2023.
"The breadth of data on our novel oncology therapies, spanning solid tumors and hematologic malignancies, underscore our commitment to addressing significant unmet medical needs in difficult-to-treat cancers," said Elcin Barker Ergun, CEO of the Menarini Group. "At Menarini Stemline, we are dedicated to driving innovation in oncology to deliver targeted therapies that bring value to people living with cancer and to the healthcare providers who care for them."
See below for full details of the Menarini Group/Stemline Therapeutics' upcoming presentations:
San Antonio Breast Cancer Symposium 2023
Lead Author | Abstract Title and ID | Presentation Details |
Bardia, A | Elacestrant vs standard-of-care in ER+/HER2- advanced or metastatic breast cancer (mBC) with ESR1 mutation: key biomarkers and clinical subgroup analyses from the phase 3 EMERALD trial 1576519/PS17-02 | December 8, 2023 7-8am CT Poster Spotlight Discussion Hemisfair Ballroom 1-2
|
Rugo, H | ELEVATE: A phase 1b/2, open-label, umbrella study evaluating elacestrant in various combinations in patients (pts) with estrogen receptor-positive (ER+), HER2-negative (HER2-) locally advanced or metastatic breast cancer (mBC) 1576517/PO2-05-04 | December 6, 2023 5-7pm CT Poster Halls 2-3 |
Ibrahim, N | ELECTRA: An open-label, multicenter, phase 1b/2 study of elacestrant in combination with abemaciclib in patients with brain metastasis (mets) from estrogen receptor-positive (ER+), HER2-negative (HER2-) breast cancer (BC) 1576518/PO2-05-05 | December 6, 2023 5-7pm CT Poster Halls 2-3
|
Patnaik, A | SUMIT-ELA: Phase 1b/2 combination of cyclin-dependent kinase 7 inhibitor (CDK7i) samuraciclib and selective estrogen receptor degrader (SERD) elacestrant in advanced hormone receptor positive (HR+) breast cancer after CDK4/6i* NA/PO3-04-13 | December 7, 2023 12-2pm CT Poster Halls 2-3
|
Bellet, M | A phase 2 randomized pre-operative, window of opportunity trial investigating the effect of elacestrant with/without triptorelin in premenopausal patients with HR+/HER2- breast cancer – SOLTI-2104-PremiÈRe trial* 1580190/PO3-19-08 | December 7, 202312-2pm CT Poster Halls 2-3
|
*Denotes investigator sponsored research or collaborative research.
65th American Society of Hematology (ASH) Annual Meeting 2023
Lead Author | Title and Publication Number | Session Name | Presentation Details |
Angelucci et al. |
Publication Number: 547
| 906. Outcomes Research – Myeloid Malignancies: Real-World Treatment Patterns and Outcomes | Oral Presentation
December 10, 2023 12:00 PM Marriott Marquis San Diego Marina, Marriott Grand Ballroom 11-13
|
Minetto et al. |
Publication Number: 2918
| 616. Acute Myeloid Leukemias: Investigational Therapies, Excluding Transplantation and Cellular Immunotherapies: Poster II | Poster Presentation
December 10, 2023 6:00 PM - 8:00 PM |
Lane et al. |
Publication Number: 4277 | 616. Acute Myeloid Leukemias: Investigational Therapies, Excluding Transplantation and Cellular Immunotherapies: Poster III
| Poster Presentation
December 11, 2023 6:00 PM - 8:00 PM |
Boichut et al. |
Publication Number: 2783 | 604. Molecular Pharmacology and Drug Resistance: Myeloid Neoplasms: Poster II | Poster Presentation
December 10, 2023 San Diego Convention Center, Halls G-H |
Navarro Vicente et al. |
Publication Number: 4234
| 613. Acute Myeloid Leukemias: Clinical and Epidemiological: Poster III | Poster Presentation
December 11, 2023 6:00 PM - 8:00 PM |
*Denotes investigator sponsored research or collaborative research
About the EMERALD Phase 3 Study (NCT03778931)
The EMERALD Phase 3 trial is a randomized, open label, active-controlled study evaluating elacestrant as second- or third-line monotherapy in ER+, HER2- advanced/mBC patients. The study enrolled 478 patients who had received prior treatment with one or two lines of endocrine therapy, including a CDK4/6 inhibitor. Patients in the study were randomized to receive either elacestrant or the investigator's choice of an approved hormonal agent. The primary endpoints of the study were progression-free survival (PFS) in the overall patient population and in patients with estrogen receptor 1 gene (ESR1) mutations. In the group of patients whose tumors had ESR1 mutations, elacestrant achieved a median PFS of 3.8 months vs 1.9 months on the SOC, and reduced the risk of progression or death by 45% (PFS HR=0.55, 95% CI: 0.39, 0.77) vs SOC.
About ORSERDU® (elacestrant)
U.S. Indication: ORSERDU (elacestrant), 345 mg tablets, is approved by the U.S. Food & Drug Administration (FDA) for the treatment of postmenopausal women or adult men with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy.
Full prescribing information for the U.S. can be found at www.orserdu.com.
Important Safety Information, ORSERDU®
Warning and Precautions
Dyslipidemia: Hypercholesterolemia and hypertriglyceridemia occurred in patients taking ORSERDU at an incidence of 30% and 27%, respectively. The incidence of Grade 3 and 4 hypercholesterolemia and hypertriglyceridemia were 0.9% and 2.2%, respectively. Monitor lipid profile prior to starting and periodically while taking ORSERDU.
Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, ORSERDU can cause fetal harm when administered to a pregnant woman. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ORSERDU and for 1 week after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ORSERDU and for 1 week after the final dose.
Adverse Reactions
Serious adverse reactions occurred in 12% of patients who received ORSERDU. Serious adverse reactions in >1% of patients who received ORSERDU were musculoskeletal pain (1.7%) and nausea (1.3%). Fatal adverse reactions occurred in 1.7% of patients who received ORSERDU, including cardiac arrest, septic shock, diverticulitis, and unknown cause (one patient each).
The most common adverse reactions (>10%), including laboratory abnormalities, of ORSERDU were musculoskeletal pain (41%), nausea (35%), increased cholesterol (30%), increased AST (29%), increased triglycerides (27%), fatigue (26%), decreased hemoglobin (26%), vomiting (19%), increased ALT (17%), decreased sodium (16%), increased creatinine (16%), decreased appetite (15%), diarrhea (13%), headache (12%), constipation (12%), abdominal pain (11%), hot flush (11%), and dyspepsia (10%).
Drug interactions
Concomitant use with CYP3A4 Inducers and/or inhibitors: Avoid concomitant use of strong or moderate CYP3A4 inhibitors with ORSERDU. Avoid concomitant use of strong or moderate CYP3A4 inducers with ORSERDU.
Use in specific populations
Lactation: Advise lactating women to not breastfeed during treatment with ORSERDU and for 1 week after the last dose.
Hepatic Impairment: Avoid use of ORSERDU in patients with severe hepatic impairment (Child-Pugh C). Reduce the dose of ORSERDU in patients with moderate hepatic impairment (Child-Pugh B).
The safety and effectiveness of ORSERDU in pediatric patients have not been established.
To report SUSPECTED ADVERSE REACTIONS, contact Stemline Therapeutics, Inc. at 1-877-332-7961 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Elacestrant is also being investigated in several clinical trials in metastatic breast cancer disease, alone or in combination with other therapies: ELEVATE (NCT05563220); ELECTRA (NCT05386108); and ELCIN (NCT05596409). Elacestrant is also being evaluated in early breast cancer disease.
About ELZONRIS® (tagraxofusp-erzs)
U.S. Indication: ELZONRIS (tagraxofusp-erzs) is a prescription medicine used to treat blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and pediatric patients 2 years and older.
Full prescribing information for the U.S. can be found at www.elzonris.com.
IMPORTANT SAFETY INFORMATION, ELZONRIS®
Boxed WARNING: CAPILLARY LEAK SYNDROME
- Capillary Leak Syndrome (CLS) which may be life-threatening or fatal, can occur in patients receiving ELZONRIS. Monitor for signs and symptoms of CLS and take actions as recommended.
Warnings and Precautions
Capillary Leak Syndrome
Capillary leak syndrome (CLS), including life-threatening and fatal cases, has been reported among patients treated with ELZONRIS. In patients receiving ELZONRIS in clinical trials, the overall incidence of CLS was 53% (65/122), including Grade 1 or 2 in 43% (52/122) of patients, Grade 3 in 7% (8/122) of patients, Grade 4 in 1% (1/122) of patients, and four fatalities (3%). The median time to onset was 4 days (range - 1 to 46 days), and all but 5 patients experienced an event in Cycle 1.
Before initiating therapy with ELZONRIS, ensure that the patient has adequate cardiac function and serum albumin is greater than or equal to 3.2 g/dL. During treatment with ELZONRIS, monitor serum albumin levels prior to the initiation of each dose of ELZONRIS and as indicated clinically thereafter, and assess patients for other signs or symptoms of CLS, including weight gain, new onset or worsening edema, including pulmonary edema, hypotension or hemodynamic instability.
Hypersensitivity Reactions
ELZONRIS can cause severe hypersensitivity reactions. In patients receiving ELZONRIS in clinical trials, hypersensitivity reactions were reported in 43% (53/122) of patients treated with ELZONRIS and were Grade ≥ 3 in 7% (9/122). Manifestations of hypersensitivity reported in ≥ 5% of patients include rash, pruritus, and stomatitis. Monitor patients for hypersensitivity reactions during treatment with ELZONRIS. Interrupt ELZONRIS infusion and provide supportive care as needed if a hypersensitivity reaction should occur.
Hepatotoxicity
Treatment with ELZONRIS was associated with elevations in liver enzymes. In patients receiving ELZONRIS in clinical trials, elevations in ALT occurred in 79% (96/122) and elevations in AST occurred in 76% (93/122). Grade 3 ALT elevations were reported in 26% (32/122) of patients. Grade 3 AST elevations were reported in 30% (36/122) and Grade 4 AST elevations were reported in 3% (4/122) of patients. Elevated liver enzymes occurred in the majority of patients in Cycle 1 and were reversible following dose interruption.
Monitor alanine aminotransferase (ALT) and aspartate aminotransferase (AST) prior to each infusion with ELZONRIS. Withhold ELZONRIS temporarily if the transaminases rise to greater than 5 times the upper limit of normal and resume treatment upon normalization or when resolved.
Adverse Reactions
Most common adverse reactions (incidence ≥ 30%) are capillary leak syndrome, nausea, fatigue, pyrexia, peripheral edema, and weight increase. Most common laboratory abnormalities (incidence ≥ 50%) are decreases in albumin, platelets, hemoglobin, calcium, and sodium, and increases in glucose, ALT and AST.
Please see full Prescribing Information, including Boxed WARNING.
To report SUSPECTED ADVERSE REACTIONS, contact Stemline Therapeutics, Inc. at 1-877-332-7961 or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
About The Menarini Group
The Menarini Group is a leading international pharmaceutical and diagnostics company, with a turnover of over $4.4 billion and over 17,000 employees. Menarini is focused on therapeutic areas with high unmet needs with products for cardiology, oncology, pneumology, gastroenterology, infectious diseases, diabetology, inflammation, and analgesia. With 18 production sites and 9 Research and Development centers, Menarini's products are available in 140 countries worldwide. For further information, please visit www.menarini.com.
About Stemline Therapeutics Inc.
Stemline Therapeutics, Inc. ("Stemline") a wholly-owned subsidiary of the Menarini Group, is a commercial-stage biopharmaceutical company focused on the development and commercialization of novel oncology therapeutics. Stemline commercializes ORSERDU® (elacestrant) in the U.S. and E.U., an oral endocrine therapy indicated for the treatment of postmenopausal women or adult men with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy. Stemline also commercializes ELZONRIS® (tagraxofusp-erzs), a novel targeted treatment directed to CD123 for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN), an aggressive hematologic cancer, in the United States and Europe, which is the only approved treatment for BPDCN in the U.S. and E.U. to date. Stemline also commercializes NEXPOVIO® (selinexor) in Europe, an XPO1 inhibitor for multiple myeloma. Stemline also has an extensive clinical pipeline of small molecules and biologics in various stages of development for a host of solid and hematologic cancers.