- Company's eTurna™ delivery platform ensures unprecedented precision, safety
- In-vivo biodistribution of mRNA into dermal cells, tissues shows high transfection efficiency, gene expression
- Data presented at ASDS Annual Meeting
MOUNTAIN VIEW, Calif., Nov. 7, 2023 /PRNewswire/ -- Turn Biotechnologies, a cell rejuvenation developer of novel mRNA medicines for untreatable, age-related conditions, announced it is the first company to safely and specifically deliver mRNA in vivo in the skin without any off-target organ uptake.
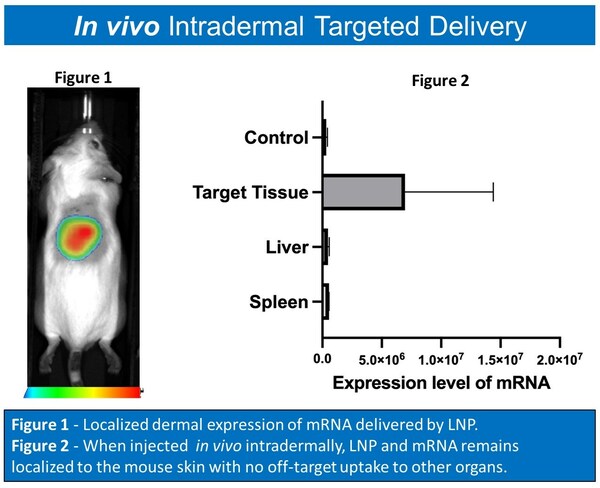
Turn Biotechnologies is the first company to safely and specifically deliver mRNA in vivo in the skin without any off-target organ uptake.
The company used its eTurna lipid nanoparticle delivery platform to precisely reach in vivo dermal fibroblasts with an mRNA formulation. The eTurna-delivered formulation remained localized in the injection site and did not distribute to other organs.
Since the skin is the body's natural layer of protection, delivering mRNA therapeutics within a lipid nanoparticle delivery system to a specific dermal target is a significant achievement. Researchers have delivered mRNA-based therapies to the lungs, spleen and liver, but no one has previously been able to target the dermal layer of the skin – the body's largest organ.
"Being the first to deliver mRNA-based therapies so precisely in skin opens the door to drug therapies that have never before been possible," said Turn Bio CEO Anja Krammer. "We are extremely excited about achieving another critical milestone on our path forward to the clinic."
Turn Bio continues to expand the breakthrough capabilities of the eTurna delivery platform by developing a library of formulations and novel lipids to deliver high transfection efficiency and selectivity for target cell and tissue types, while maintaining low-to-no cytotoxicity and immunogenicity.
Turn Bio's most recent research was shared at the American Society for Dermatologic Surgery Annual Meeting held Nov. 2-5 in Chicago.
"Turn Bio's achievement is extremely exciting for dermatologic medicine as skin is the most challenging organ to treat effectively and safely," said Dr. Amelia Hausauer, director of dermatology at Aesthetx Plastic Surgery and Dermatology in Campbell, California, and chair of the ASDS Regenerative Medicine Session. "It shows promise to significantly expand the therapies dermatologists can provide – and dramatically improve the quality of our patients' lives."
ABOUT TURN BIOTECHNOLOGIES
Turn Bio is a pre-clinical-stage company focused on repairing tissue at the cellular level and developing transformative drug delivery systems. The company's proprietary mRNA-based ERA™ reprogramming technology restores optimal gene expression by combatting the effects of aging in the epigenome. This restores cells' ability to prevent or treat disease and heal or regenerate tissue. It will help to fight incurable chronic diseases. Its eTurna™ delivery platform uses unique formulations to precisely deliver cargo to specific organs, tissues, and cell types.
The company is completing pre-clinical research on tailored therapies targeting indications in dermatology and immunology, and developing therapies for ophthalmology, osteo-arthritis, and the muscular system. For more information, see www.turn.bio.
FOR MORE INFORMATION, CONTACT:
Jim Martinez, rightstorygroup
jim@rightstorygroup.com or (312) 543-9026
Photo - https://mma.prnasia.com/media2/2266341/TURN_BIO_INVIVO_BIODISTRIBUTION.jpg?p=medium600










