MELBOURNE, Australia, Sept. 13, 2022 /PRNewswire/ -- Cabazitaxel (Jevtana, Sanofi) is a market leading chemotherapy for the treatment of advanced prostate cancer (mCRPC). Developed by Australian biotech company Starpharma, DEP® cabazitaxel is a patented, highly water soluble dendrimer nanoparticle version of standard cabazitaxel which has shown, in preclinical and clinical studies, benefits in terms of safety and efficacy.
In a poster presentation at ESMO[1] (European Society of Medical Oncology) by Principal investigator of the Starpharma trial[2], Professor Robert Jones of the Velindre Cancer Centre in Wales, exciting new data on the superior efficacy and lower incidence of key side effects in mCRPC patients was presented.
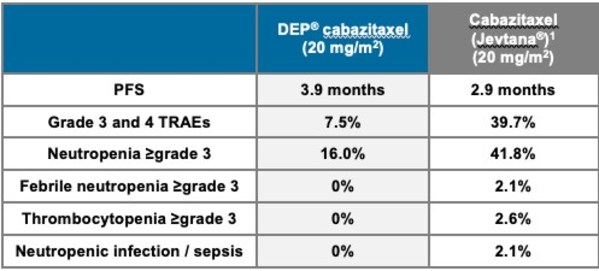
DEP® cabazitaxel showed multiple potential benefits for patients with mCRPC, including:
- A >30% improvement in median progression free survival (PFS; time a patient lives without disease progression following treatment) compared to standard cabazitaxel - 3.9 months v 2.9 months respectively
- 100% of evaluable DEP® cabazitaxel patients achieved a response in at least 1 measure of efficacy (soft tissue disease, prostate specific antigen and/or bone disease)
- Lower incidence of severe (Grade 3 or 4) treatment related adverse events (TRAEs) compared to standard cabazitaxel - 7.5% v 39.7% respectively
- No severe hypersensitivity reactions observed, or steroid pre-medication required, and only 2 patients required prophylactic G-CSF (used after chemotherapy to help white blood cells recover) in contrast to standard cabazitaxel.
Patients enrolled had an average age of 73 and were heavily pre-treated before entering the study (average of 4 other cancer treatment types - 70 cycles/months), in addition to surgery and radiation. 96% had also previously received either docetaxel and/or standard cabazitaxel (Jevtana). This age group and level of pre-treatment is important to note because these patients would not be expected to respond as well to further similar therapies and are at higher risk of neutropenic complications.
Starpharma CEO, Dr Jackie Fairley, commented: "These results show DEP® cabazitaxel achieved both a longer duration of PFS and fewer severe side effects compared to published data on Jevtana®, illustrating the potential for DEP® cabazitaxel to provide better outcomes for mCRPC patients."
[1] Jones, RH, et al., ESMO 2022 Congress, FPN 1403P. |
[2] Twenty-five patients with mCRPC were enrolled in this cohort across five trial sites in the UK and Australia. Trial participants received DEP® cabazitaxel every 21 days, repeated for up to 12 cycles. |